
Parylene exhibits excellent biocompatibility and has been certified by the U.S. FDA, meeting the USP Class VI standards for biomedical materials, making it a suitable material for long-term implantation in the body. Due to its excellent moisture resistance, chemical barrier properties, dielectric properties, dry film lubrication, and biocompatibility, it is widely used as a surface coating for biomedical devices in international clinical applications.Current Applications of Parylene Coatings in the Medical Device Industry:
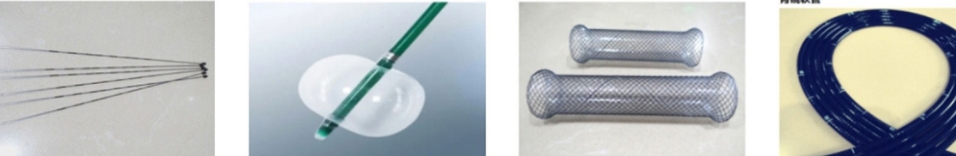
Therapeutic and Diagnostic Medical Products: Ablation needles, puncture needles, neural probes, endoscopic tubing, etc. Implantable Medical Devices: Parylene coatings provide ideal surface modification for implantable medical devices such as coronary stents, neural stimulators, cochlear implants, ocular implants, and pacemakers. They protect medical devices and components and are recognized for their biocompatibility with tissue-contacting surfaces.
Medical Elastomer Products: Medical-grade silicone and rubber products (e.g., catheters, medical seals, infusion components) require highly flexible coatings. Parylene coatings meet these requirements, reduce friction coefficients, eliminate surface tackiness, and prevent discoloration and contaminant buildup.
Medical Molding Equipment: The dry film lubricity of Parylene coatings makes them ideal release agents for molds and molding equipment (e.g., mandrels), eliminating flaking and delamination. This significantly enhances the safety and efficacy of these components. Parylene coatings are inert solids, thus leaving no residue that could contaminate molded products.
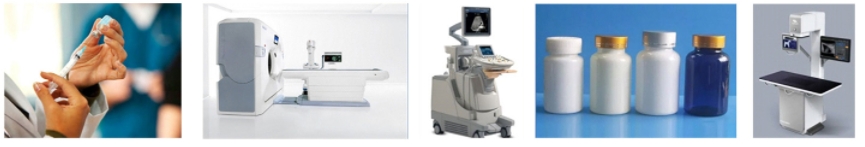
Medical Electronic Devices: Parylene coatings protect medical electronic components from moisture, biological fluids, bio-gases, and sterilization processes, all of which can cause premature device failure. The protection provided by Parylene coatings not only extends the lifespan of devices and avoids costly repairs but also reduces the risk of failure during critical periods. This is applicable to various technologies, including electromechanical and electrosurgical devices, infusion and fluid heating technologies, robotic surgical systems, and ultrasound and X-ray imaging platforms.
Pharmaceutical Containers: Whether the application requires barrier capabilities or dry film lubrication, Parylene coatings benefit pre-filled syringes and pharmaceutical containers. Parylene coatings, applied at micron-level thicknesses, can prevent the leaching and extraction of substances when the substrate comes into contact with medications. Additionally, since the static and dynamic coefficients of friction of the inert Parylene coating are nearly identical, the initial force required to use the container is eliminated, enhancing its usability.
Application Scenario Classification
1. Implantable Medical Devices
· Long-term implants: pacemakers, vascular stents, neural stimulation electrodes, etc.
· Short-term/temporary implants: ablation needles, catheters, guidewires, etc.
2. Wearable Medical Devices
· Smart wristbands, blood glucose monitoring patches, etc. (contact with body fluids such as sweat and blood)
3. Non-protective Equipment
· Medical tools for use in constant temperature and humidity environments (typically not requiring parylene coating)
Key Product Requirements
● Medical-grade safety standards: Comply with ISO 10993 biocompatibility certification (cytotoxicity, sensitization, and irritation testing)
● Long-term stability: Maintain performance in the presence of body fluids (blood, sweat, urine), with no risk of degradation or failure
● Functional Requirements:
○ Insulation (e.g., leakage protection for cosmetic and puncture needles)
○ Lubricity (e.g., smooth surface treatment of endoscopic catheters and guidewires to reduce tissue damage)
○ Corrosion resistance (resists long-term erosion by body fluids)
○ Barrier protection (prevents biological contamination or metal ion release)
Environmental Challenges
● Body fluid contact: Blood (pH 7.35-7.45), sweat (salty and slightly acidic), urine (pH 4.6-8.0)
● Mechanical friction: Repeated movement of catheters and guidewires within the human body may cause coating wear
● Sterilization compatibility: Must withstand sterilization methods such as high temperature and autoclave, ethylene oxide (EO), and gamma irradiation
Parylene DM / Parylene N Series (Halogen-Free)
● Parylene DM Series (Preferred):
○ Excellent biocompatibility (FDA-approved)
○ Ultra-thin, uniform coating (0.1-50μm) without compromising device precision
○ Chemical resistance and long-term stability (>10 years of implant validation)
○ Optimized lubricity (friction coefficient <0.2, reducing the risk of tissue damage)
● Parylene N Series (cost-effective alternative):
○ Meets the needs of disposable products (such as catheters and puncture needles)
○ Offers significant cost advantages while maintaining basic protective performance
Do you have a question and would you like to get in touch with our experts? We look foward to receiving your inquiry!